BASIC CHEMISTRY - OFFLINE 1.0.1
Free Version
Publisher Description
BASIC CHEMISTRY - OFFLINE - COMPLITELY FREE - BASIC CONCEPT OF CHEMISTRY
BASIC CHEMISTRY - KNOWLEDGE ON THE GO - NO ADS
Through this KNOWLEDGE ON THE GO series by APPSPHINX LEARNING, we bring highly sophisticated and well-organized fundamental concepts of chemistry (Courtesy OpenStax) while addressing the needs of students with diverse backgrounds and learning styles. Each topic builds upon previously developed material to demonstrate the cohesiveness and structure of chemistry.
👉 AMAZING FEATURES
✔ 100% FREE
✔ NO ADS
✔ 100% OFFLINE
✔ CAREFULLY CRAFTED ALL HIGH-QUALITY CHEMISTRY MATERIAL
✔ NIGHT MODE OPTION
✔ APART FROM THE SCHOOL STUDENTS, THIS APP IS SUITABLE FOR, ENGINEERING, UPSC CSE, SSC CGL, IBPS - BANK PO, CAT, OPSC & ASO ASPIRANT WHO WANTS TO CLEAR THEIR BASIC CHEMISTRY CONCEPT.
👉DETAIL APP CONTENT
✔ 1: Essential Ideas9
1.1 Chemistry in Context
1.2 Phases and Classification of Matter
1.3 Physical and Chemical Properties
1.4 Measurements
1.5 Measurement Uncertainty, Accuracy, and Precision
1.6 Mathematical Treatment of Measurement Results
✔ 2: Atoms, Molecules, and Ions
2.1 Early Ideas in Atomic Theory
2.2 Evolution of Atomic Theory
2.3 Atomic Structure and Symbolism
2.4 Chemical Formulas
2.5 The Periodic Table
2.6 Molecular and Ionic Compounds
2.7 Chemical Nomenclature
✔ 3: Composition of Substances and Solutions
3.1 Formula Mass and the Mole Concept
3.2 Determining Empirical and Molecular Formulas
3.3 Molarity
3.4 Other Units for Solution Concentrations
✔ 4: Stoichiometry of Chemical Reactions
4.1 Writing and Balancing Chemical Equations
4.2 Classifying Chemical Reactions
4.3 Reaction Stoichiometry
4.4 Reaction Yields
4.5 Quantitative Chemical Analysis
✔ 5: Thermochemistry
5.1 Energy Basics
5.2 Calorimetry
5.3 Enthalpy
✔ 6: Electronic Structure and Periodic Properties of Elements
6.1 Electromagnetic Energy
6.2 The Bohr Model
6.3 Development of Quantum Theory
6.4 Electronic Structure of Atoms (Electron Configurations)
6.5 Periodic Variations in Element Properties
✔ 7: Chemical Bonding and Molecular Geometry
7.1 Ionic Bonding
7.2 Covalent Bonding
7.3 Lewis Symbols and Structures
7.4 Formal Charges and Resonance
7.5 Strengths of Ionic and Covalent Bonds
7.6 Molecular Structure and Polarity
✔ 8: Advanced Theories of Covalent Bonding
8.1 Valence Bond Theory
8.2 Hybrid Atomic Orbitals
8.3 Multiple Bonds
8.4 Molecular Orbital Theory
✔ 9: Gases
9.1 Gas Pressure
9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
9.3 Stoichiometry of Gaseous Substances, Mixtures, and Reactions
9.4 Effusion and Diffusion of Gases
9.5 The Kinetic-Molecular Theory
9.6 Non-Ideal Gas Behavior
✔ 10: Liquids and Solids
10.1 Intermolecular Forces
10.2 Properties of Liquids
10.3 Phase Transitions
10.4 Phase Diagrams
10.5 The Solid State of Matter
10.6 Lattice Structures in Crystalline Solids
✔ 11: Solutions and Colloids
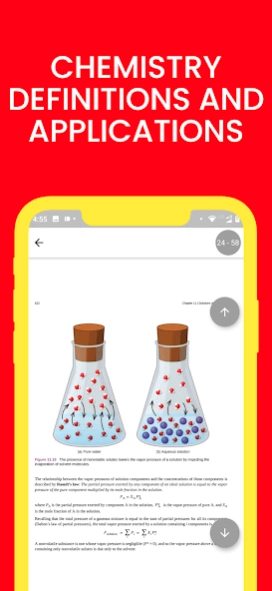
11.1 The Dissolution Process
11.2 Electrolytes
11.3 Solubility
11.4 Colligative Properties
11.5 Colloids
✔ 12: Kinetics
12.1 Chemical Reaction Rates
12.2 Factors Affecting Reaction Rates
12.3 Rate Laws
12.4 Integrated Rate Laws
12.5 Collision Theory
12.6 Reaction Mechanisms
12.7 Catalysis
✔ 13: Fundamental Equilibrium Concepts
13.1 Chemical Equilibria
13.2 Equilibrium Constants
13.3 Shifting Equilibria: Le Châtelier’s Principle
13.4 Equilibrium Calculations
✔ 14: Acid-Base Equilibria
14.1 Brønsted-Lowry Acids and Bases
14.2 pH and pOH
14.3 Relative Strengths of Acids and Bases
14.4 Hydrolysis of Salts
14.5 Polyprotic Acids
14.6 Buffers
14.7 Acid-Base Titrations
✔ 15: Equilibria of Other Reaction Classes
15.1 Precipitation and Dissolution
15.2 Lewis Acids and Bases
15.3 Coupled Equilibria
✔ 16: Thermodynamics
16.1 Spontaneity
16.2 Entropy
16.3 The Second and Third Laws of Thermodynamics
16.4 Free Energy
✔ 17: Electrochemistry
17.1 Review of Redox Chemistry
17.2 Galvanic Cells
17.3 Electrode and Cell Potentials
17.4 Potential, Free Energy, and Equilibrium
17.5 Batteries and Fuel Cells
17.6 Corrosion
17.7 Electrolysis
About BASIC CHEMISTRY - OFFLINE
BASIC CHEMISTRY - OFFLINE is a free app for Android published in the Teaching & Training Tools list of apps, part of Education.
The company that develops BASIC CHEMISTRY - OFFLINE is Appsphinx Learning. The latest version released by its developer is 1.0.1.
To install BASIC CHEMISTRY - OFFLINE on your Android device, just click the green Continue To App button above to start the installation process. The app is listed on our website since 2021-02-23 and was downloaded 2 times. We have already checked if the download link is safe, however for your own protection we recommend that you scan the downloaded app with your antivirus. Your antivirus may detect the BASIC CHEMISTRY - OFFLINE as malware as malware if the download link to com.appsphinx.basicchemistry is broken.
How to install BASIC CHEMISTRY - OFFLINE on your Android device:
- Click on the Continue To App button on our website. This will redirect you to Google Play.
- Once the BASIC CHEMISTRY - OFFLINE is shown in the Google Play listing of your Android device, you can start its download and installation. Tap on the Install button located below the search bar and to the right of the app icon.
- A pop-up window with the permissions required by BASIC CHEMISTRY - OFFLINE will be shown. Click on Accept to continue the process.
- BASIC CHEMISTRY - OFFLINE will be downloaded onto your device, displaying a progress. Once the download completes, the installation will start and you'll get a notification after the installation is finished.